
Calibrations Customer Survey (external link).It may have either absorption or emission line spectrum based on whether the atom is light or another colliding electron. Since no two elements generate the same spectral lines, the specific elements line spectrum can differentiate them. Each element generates a unique set of spectral lines.
#Atomic spectra graph series#
A series of coloured lines is known as a line or atomic spectra. And the emitted light is observed as a series of coloured lines with dark spaces in between. When atoms are excited, they emit light from specific wavelengths corresponding to different colours. Why should atomic spectra be a pure line spectrum?Īnswer. If the electron drops in the energy level, a photon emits resulting in the emission line and, when the electron absorbs a photon and increase in the energy level, an absorption line is visible on the spectrum. Atomic spectrum lines relate to electron transitions between the energy levels. How do the lines on the atomic spectrum relate to the transition of electrons between energy levels?Īnswer. Therefore, the wavelength of the light emitted can help to determine which element has produced the light. The energy levels in the atom are unique to each element on the periodic table. The electrons that drop from the higher energy levels to the lower energy levels in an atom release a photon with a specific wavelength, which generates the atomic emission spectrum. Explain how the atomic emission spectra occur and how they relate to the elements on the periodic table.Īnswer.
#Atomic spectra graph free#
The principle of atomic absorption spectroscopy uses the fact that free electrons emitted in an atomizer can absorb radiation at a specific frequency. Also, atomic emissions can explain this electronic transformation.Ītomic absorption spectroscopy: for absorption, there should be an identical energy variation between the lower and higher energy levels. There are three kinds of atomic spectroscopy, they are Ītomic emission spectroscopy: It requires the transition of energy from the ground to the excited state.
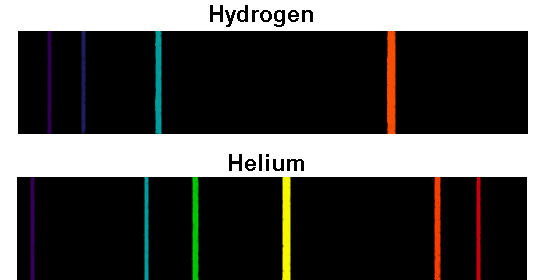
Atomic SpectroscopyĪtomic spectroscopy is a study of electromagnetic radiation that is absorbed or released from atoms. Single-electron atoms such as hydrogen have spectral series of Z = 1. It is easy to measure the spectral lines using the Rydberg formula. In cgs, where “me” is electron mass, “e” is the charge of the electron, h-bar, “Z” is the atomic number, and “n” is the principal quantum number of the electron state. It is a unit of energy, explained in terms of the ground-state energy of the electron in the Bohr model of the hydrogen atom. The equation is a generalisation of the Balmer series for all atomic hydrogen transitions. In atomic physics, Rydberg’s equation calculates the wavelength of the spectral line in a wide range of chemical elements.

2 FAQs about Atomic Spectra Characteristics of Atomic Spectra


 0 kommentar(er)
0 kommentar(er)
